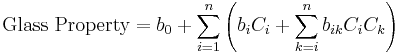Calculation of glass properties
The calculation of glass properties (glass modeling) is used to predict glass properties of interest or glass behavior under certain conditions (e.g., during production) without experimental investigation, based on past data and experience, with the intention to save time, material, financial, and environmental resources, or to gain scientific insight. It was first practised at the end of the 19th century by A. Winkelmann and O. Schott. The combination of several glass models together with other relevant functions can be used for optimization and six sigma procedures. In the form of statistical analysis glass modeling can aid with accreditation of new data, experimental procedures, and measurement institutions (glass laboratories).
Contents |
History
Historically, the calculation of glass properties is directly related to the founding of glass science. At the end of the 19th century the physicist Ernst Abbe developed equations that allow calculating the design of optimized optical microscopes in Jena, Germany, stimulated by co-operation with the optical workshop of Carl Zeiss. Before Ernst Abbe's time the building of microscopes was mainly a work of art and experienced craftsmanship, resulting in very expensive optical microscopes with variable quality. Now Ernst Abbe knew exactly how to construct an excellent microscope, but unfortunately, the required lenses and prisms with specific ratios of refractive index and dispersion did not exist. Ernst Abbe was not able to find answers to his needs from glass artists and engineers; glass making was not based on science at this time.[2]
In 1879 the young glass engineer Otto Schott sent Abbe glass samples with a special composition (lithium silicate glass) that he had prepared himself and that he hoped to show special optical properties. Following measurements by Ernst Abbe, Schott's glass samples did not have the desired properties, and they were also not as homogeneous as desired. Nevertheless, Ernst Abbe invited Otto Schott to work on the problem further and to evaluate all possible glass components systematically. Finally, Schott succeeded in producing homogeneous glass samples, and he invented borosilicate glass with the optical properties Abbe needed.[2] These inventions gave rise to the well-known companies Zeiss and Schott Glass (see also Timeline of microscope technology). Systematic glass research was born. In 1908, Eugene Sullivan founded glass research also in the United States (Corning, New York).[3]
At the beginning of glass research it was most important to know the relation between the glass composition and its properties. For this purpose Otto Schott introduced the additivity principle in several publications for calculation of glass properties.[4][5][6] This principle implies that the relation between the glass composition and a specific property is linear to all glass component concentrations, assuming an ideal mixture, with Ci and bi representing specific glass component concentrations and related coefficients respectively in the equation below. The additivity principle is a simplification and only valid within narrow composition ranges as seen in the displayed diagrams for the refractive index and the viscosity. Nevertheless, the application of the additivity principle lead the way to many of Schott’s inventions, including optical glasses, glasses with low thermal expansion for cooking and laboratory ware (Duran), and glasses with reduced freezing point depression for mercury thermometers. Subsequently, English[7] and Gehlhoff et al.[8] published similar additive glass property calculation models. Schott’s additivity principle is still widely in use today in glass research and technology.[9][10]
- Additivity Principle:
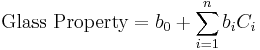
Global models
Schott and many scientists and engineers afterwards applied the additivity principle to experimental data measured in their own laboratory within sufficiently narrow composition ranges (local glass models). This is most convenient because disagreements between laboratories and non-linear glass component interactions do not need to be considered. In the course of several decades of systematic glass research thousands of glass compositions were studied, resulting in millions of published glass properties, collected in glass databases. This huge pool of experimental data was not investigated as a whole, until Bottinga,[13], Kucuk[14], Priven[15], Choudhary[16], Mazurin[17], and Fluegel[18][19] published their global glass models, using various approaches. In contrast to the models by Schott the global models consider many independent data sources, making the model estimates more reliable. In addition, global models can reveal and quantify non-additive influences of certain glass component combinations on the properties, such as the mixed-alkali effect as seen in the diagram on the right, or the boron anomaly. Global models also reflect interesting developments of glass property measurement accuracy, e.g., a decreasing accuracy of experimental data in modern scientific literature for some glass properties, shown in the diagram. They can be used for accreditation of new data, experimental procedures, and measurement institutions (glass laboratories). In the following sections (except melting enthalpy) empirical modeling techniques are presented, which seem to be a successful way for handling huge amounts of experimental data. The resulting models are applied in contemporary engineering and research for the calculation of glass properties.
Non-empirical (deductive) glass models exist.[20] They are often not created to obtain reliable glass property predictions in the first place (except melting enthalpy), but to establish relations among several properties (e.g. atomic radius, atomic mass, chemical bond strength and angles, chemical valency, heat capacity) to gain scientific insight. In future, the investigation of property relations in deductive models may ultimately lead to reliable predictions for all desired properties, provided the property relations are well understood and all required experimental data are available.
Methods
Glass properties and glass behavior during production can be calculated through statistical analysis of glass databases such as GE-SYSTEM[21] SciGlass[22] and Interglad,[23] sometimes combined with the finite element method. For estimating the melting enthalpy thermodynamic databases are used.
Linear regression
If the desired glass property is not related to crystallization (e.g., liquidus temperature) or phase separation, linear regression can be applied using common polynomial functions up to the third degree. Below is an example equation of the second degree. The C-values are the glass component concentrations like Na2O or CaO in percent or other fractions, the b-values are coefficients, and n is the total number of glass components. The glass main component silica (SiO2) is excluded in the equation below because of over-parametrization due to the constraint that all components sum up to 100%. Many terms in the equation below can be neglected based on correlation and significance analysis. Systematic errors such as seen in the picture are quantified by dummy variables. Further details and examples are available in an online tutorial by Fluegel.[24]
Non-linear regression
The liquidus temperature has been modeled by non-linear regression using neural networks[26] and disconnected peak functions.[25] The disconnected peak functions approach is based on the observation that within one primary crystalline phase field linear regression can be applied[27] and at eutectic points sudden changes occur.
Glass melting enthalpy
The glass melting enthalpy reflects the amount of energy needed to convert the mix of raw materials (batch) to a melt glass. It depends on the batch and glass compositions, on the efficiency of the furnace and heat regeneration systems, the average residence time of the glass in the furnace, and many other factors. A pioneering article about the subject was written by Carl Kröger in 1953.[28]
Finite element method
For modeling of the glass flow in a glass melting furnace the finite element method is applied commercially,[29][30] based on data or models for viscosity, density, thermal conductivity, heat capacity, absorption spectra, and other relevant properties of the glass melt. The finite element method may also be applied to glass forming processes.
Optimization
It is often required to optimize several glass properties simultaneously, including production costs. GE-SYSTEM[21] [31] This can be performed, e.g., by simplex search, or in a spreadsheet as follows:
- Listing of the desired properties;
- Entering of models for the reliable calculation of properties based on the glass composition, including a formula for estimating the production costs;
- Calculation of the squares of the differences (errors) between desired and calculated properties;
- Reduction of the sum of square errors using the Solver option[32] in Microsoft Excel with the glass components as variables. Other software (e.g. Microcal Origin) can also be used to perform these optimizations.
It is possible to weight the desired properties differently. Basic information about the principle can be found in an article by Huff et al.[33] The combination of several glass models together with further relevant technological and financial functions can be used in six sigma optimization.
See also
References
- ^ Calculation of the Refractive Index of Glasses
- ^ a b Vogel, Werner (1994). Glass chemistry (2nd revised ed.). Berlin: Springer-Verlag. ISBN 3-540-57572-3.
- ^ Eugene Sullivan and Corning Glass Works
- ^ Winkelmann A., Schott O. (1894). "Über die Elastizität und über die Druckfestigkeit verschiedener neuer Gläser in ihrer Abhängigkeit von der chemischen Zusammensetzung". Ann. Physik Chemie 51: 697.
- ^ Winkelmann A., Schott O. (1894). "Über thermische Widerstandscoefficienten verschiedener Gläser in ihrer Abhängigkeit von der chemischen Zusammensetzung". Ann. Physik Chemie 51: 730.
- ^ Winkelmann A., Schott O. (1893). "Über die specifischen Wärmen verschieden zusammengesetzter Gläser". Ann. Physik Chemie 49: 401.
- ^ English S. (1924). "The effect of composition on the viscosity of glass. Part II". J. Soc. Glass Technol. 8: 205–48.
"... Part III Some Four-component Glasses". J. Soc. Glass Technol. 9: 83–98. 1925.
"...Part IV. Calculation of the Influence of Minor Constituents". J. Soc. Glass Technol. 10: 52–66. 1926. - ^ Gehlhoff G., Thomas M. (1925). Z. Techn. Physik (6): 544.; Z. Techn. Physik (7): 105, 260. 1926.; "Lehrbuch der technischen Physik", J. A. Barth-Verlag, Leipzig, 1924, p 376.
- ^ Lakatos T., Johansson L.G., Simmingsköld B. (June 1972). "Viscosity temperature relations in the glass system SiO2-Al2O3-Na2O-K2O-CaO-MgO in the composition range of technical glasses". Glass Technology 13 (3): 88–95.
- ^ Terese Vascott; Thomas P. Seward III (2005). High Temperature Glass Melt Property Database for Process Modeling. Wiley-American Ceramic Society. ISBN 1-57498-225-7.
- ^ The Mixed-Alkali Effect for the Viscosity of Glasses
- ^ a b Overview, Measurement Errors of Glass Properties
- ^ Bottinga Y., Weill D.F. (May 1972). "The viscosity of magmatic silicate liquids: a model for calculation". Am. J. Sci. 272 (5): 438–75. doi:10.2475/ajs.272.5.438.
- ^ Kucuk A., Clare A. G, Jones L. (October 1999). "An estimation of the surface tension of silicate glass melts at 1400 °C using statistical analysis". Glass Technol. 40 (5): 149–53.
- ^ Priven A.I. (December 2004). "General Method for Calculating the Properties of Oxide Glasses and Glass-Forming Melts from their Composition and Temperature". Glass Technology 45 (6): 244–54. http://www.sciglass.info/Publications/Priven.pdf.
- ^ M. K. Choudhary, R. M. Potter (2005). "9. Heat Transfer in Glass-Forming Melts". In Angelo Montenero; Pye, David; Innocent Joseph. Properties of Glass-Forming Melts. Boca Raton: CRC. ISBN 1-57444-662-2. http://books.google.com/books?id=oFs1ImozZW4C&pg=PA249.
- ^ O. V. Mazurin, O. A. Prokhorenko: "Electrical conductivity of glass melts"; Chapter 10 in: "Properties of Glass-Forming Melts" ed. by D. L. Pye, I. Joseph, A. Montenaro; CRC Press, Boca Raton, Florida, 2005, ISBN 1-57444-662-2.
- ^ Fluegel A. (2007). "Glass Viscosity Calculation Based on a Global Statistical Modeling Approach" (PDF). Glass Technol.: Europ. J. Glass Sci. Technol. A 48 (1): 13–30. http://glassproperties.com/viscosity/Viscosity_2006_AFluegel.pdf.
- ^ Fluegel, Alexander (2007). "Global Model for Calculating Room-Temperature Glass Density from the Composition". Journal of the American Ceramic Society 90 (8): 2622. doi:10.1111/j.1551-2916.2007.01751.x.
- ^ Milos B. Volf: "Mathematical Approach to Glass" Glass Science and Technology, vol. 9, Elsevier, 1988, ISBN 0-444-98951-X
- ^ a b GE-SYSTEM
- ^ SciGlass
- ^ Interglad
- ^ A. Fluegel: Statistical Regression Modeling of Glass Properties - A Tutorial
- ^ a b Glass Liquidus Temperature Calculation using disconnected peak functions
- ^ Dreyfus, C (2003). "A machine learning approach to the estimation of the liquidus temperature of glass-forming oxide blends". Journal of Non-Crystalline Solids 318: 63. doi:10.1016/S0022-3093(02)01859-8.
- ^ Hanni J.B., Pressly E., Crum J.V., Minister K.B.C., Tran D., Hrma P., Vienna J.D. (2005). "Liquidus temperature measurements for modeling oxide glass systems relevant to nuclear waste vitrification". Journal of Materials Science 20 (12): 3346–57. doi:10.1557/JMR.2005.0424.
- ^ Kröger, Carl (1953). "Theoretischer Wärmebedarf der Glasschmelzprozesse (Theoretical heat demand of glass melting processes)" (in German). Glastechnische Berichte 26 (7): 202–14.
- ^ Glass Service, Furnace Design
- ^ Brochure: Flow modeling software for the glass industry, Fluent Inc.
- ^ Glass property optimization
- ^ Excel Solver
- ^ Huff, N. T.; Call, A. D. (1973). "Computerized Prediction of Glass Compositions from Properties". Journal of the American Ceramic Society 56 (2): 55. doi:10.1111/j.1151-2916.1973.tb12356.x.
|
|||||||||||||||||||||||